Safety Rules While Handling Acids
Work Practices:
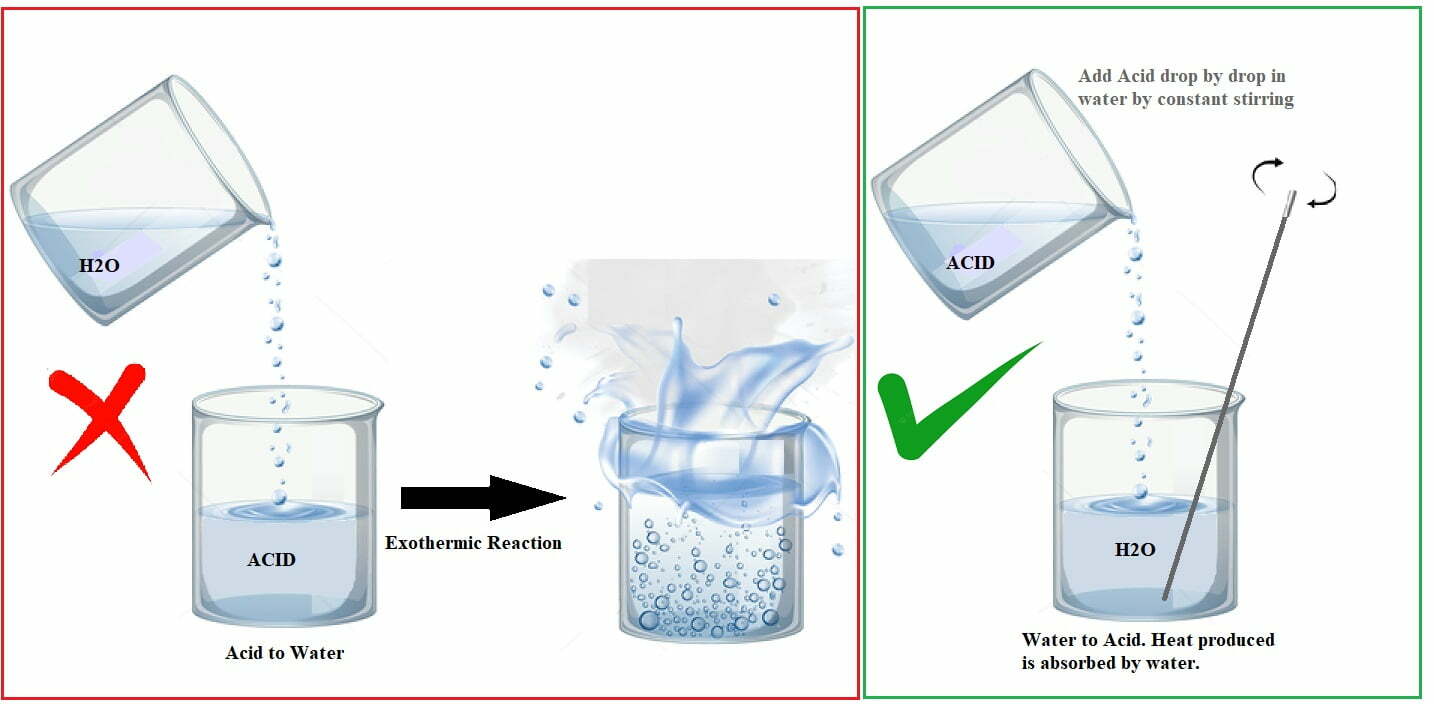
- Always remember the 3 ‘A’s of acid handling – Always Add Acid to water or base.
- Always treat any liquid in chemical area as acid because few acids like HF may look and feel like water.
- All spills and leakage should be cleaned up immediately.
- Safety equipment such as fire extinguishers, Eye wash basin and safety showers should be kept clean and readily accessible.
- Open bottles or other acid/base containers slowly and carefully, and wear protective equipment to guard hands, face, and body from splashes, vapors, gasses and fumes.
- Wipe drips from containers and bench tops. Skin contact with dry residue will result in burns.
- Do not mix acids with solvents or flammables. Special care should be taken while draining either of the products to ensure no reaction takes place.
- Do not drain acids directly. Do not interchange the bottle caps. Put the same cap back on the bottle making sure that it is tight.
- Store all acids in a dedicated corrosives or acid cabinet. The best acid cabinets are built with wood because metal cabinets will quickly corrode from acid fumes.
- Always keep the appropriate color-coded acid bottle cap on the concentrated acid bottle. If an acid bottle cap becomes cracked or discolored, always replace the cap with the proper color-coded cap.
Acid Effects:
- Concentrated hydrochloric acid fumes are responsible for most of the corrosion damage in your chemical storeroom. Storing hydrochloric acid in a wood acid cabinet is a must. Hydrochloric acid fumes will quickly corrode metal cabinets.
- Nitric acid is a strong oxidizing agent. Concentrated nitric acid must be stored in a separate liquid-tight compartment within an acid cabinet. If nitric acid is mixed with a flammable organic compound, such as acetic acid, the heat from the oxidation and neutralization reactions is enough to ignite the flammable material. Nitric acid may also turn yellow over time because of the release of nitrogen dioxide on exposure to light. The yellow color does not affect the product’s usefulness in the school laboratory.
- Glacial acetic acid is a flammable liquid. It should be stored in an acid cabinet, but in a location that is isolated from possible contact with nitric acid. Glacial acetic acid freezes at 16.6 °C; the material may crystallize in a cool storeroom. If this occurs, allow the bottle to warm up to ambient (25 °C) temperature.
- Concentrated sulfuric acid is a strong dehydrating agent. Because of its strong ability to remove water, it reacts violently with many organic materials such as sugar, wood and paper. If sulfuric acid has turned brown, it has probably been contaminated with an organic material and its purity should be in question.
- Concentrated phosphoric acid is hygroscopic and will absorb water over time. Keep tightly sealed.
PPEs:

- Nitrile rubber gloves are acid-resistant, used when handling concentrated acids.
- Chemical splash goggles must be worn whenever acids or acid solutions are used. Safety glasses are not adequate protection.
- Good ventilation should be available whenever hydrochloric, nitric or acetic acids are used.
- Spill control materials (sand, absorbent and neutralizer) must be available whenever acids or acid solutions are used.
First Aid:
- Cool the burn under cold running water for at least 20 minutes. This will help to cool the burn and wash out the chemical. Only use water, do not rub or wipe. If you don’t have access to water, you can use other harmless liquids (eg. milk/curd).
- If an acid is splashed in the eyes, use an eyewash to wash the eyes for at least 15–20 minutes. Make sure the eyelids are held open to properly flush them. Ask the victim to look up, down and sideways to better reach all parts of the eye.
- If an acid is splashed onto clothing, Try to remove the chemical and contaminated clothing from contact with the skin and eyes, but be very careful not to touch or spread the chemical. Use gloves to cover hands and if possible carefully cut away clothing, rather than pulling them off over the head. Do not wipe skin as this may spread contamination.
- If acid is ingested, do not induce vomiting, do not try to neutralize the acid with a strong base, and do not give the victim any sodium bicarbonate or any carbonated drinks. If the victim is conscious, immediately have the victim rinse their mouth out with water. Have the victim drink one or two cups of water or milk. Call a poison control center or hospital emergency room and follow their directions.
- Always seek professional medical attention upon exposure to any hazardous chemical, especially concentrated acids.
